Driving new AFib treatments by identifying its source
A heart physician and a scientist are gaining momentum in the quest to understand and treat atrial fibrillation, a common heart condition with devastating effects on millions across the globe.
Vadim Fedorov, PhD, was only six years old when his grandfather was hospitalized with a heart attack in their hometown of Voronezh, Russia, but can recall the incident in vivid detail.
“Sitting at my grandfather’s bedside in the hospital, I promised him that someday, I would find a way to make his heart beat forever,” Fedorov says.
Fedorov’s grandfather eventually recovered, but he lost his father and grandmother to sudden cardiac arrest in the years that followed. He enrolled in a Physics of Living Systems program at the Moscow Institute of Physics and Technology, resolved to develop technology that could help prevent cardiac death for families like his. And that’s exactly what he did.
Since he joined the Ohio State College of Medicine Department of Physiology and Cell Biology in 2011, Vadim Fedorov, PhD, the Corrine Frick Research Chair in Heart Failure and Arrythmia, has conducted pioneering research in cardiac arrhythmia, a condition that occurs when the electrical impulses that coordinate heart rhythms misfire, causing the heart to beat too quickly or too slowly. If not treated, cardiac arrhythmia can be fatal.
Not long after he joined the faculty, he met a kindred spirit in John Hummel, MD, an electrophysiologist at The Ohio State University Wexner Medical Center and professor in the College of Medicine. A specialist in the treatment of cardiac arrhythmia, particularly an arrhythmia called atrial fibrillation (commonly known as AFib), Dr. Hummel is always looking for new strategies to improve patient outcomes and quality of life.
His initial meeting with Fedorov would prove to be fortuitous. For much of the past decade, the two have worked together to uncover the hidden sources of atrial fibrillation and pave the way to more effective treatment for millions.

Ending the debate
AFib is a rapid, irregular heartbeat that originates from the two upper chambers of the heart called the atria. Instead of coordinating with the ventricles — the two lower chambers — to maintain a steady heartbeat, the atria essentially go rogue. Untreated, AFib can lead to blood clots, stroke and heart failure.
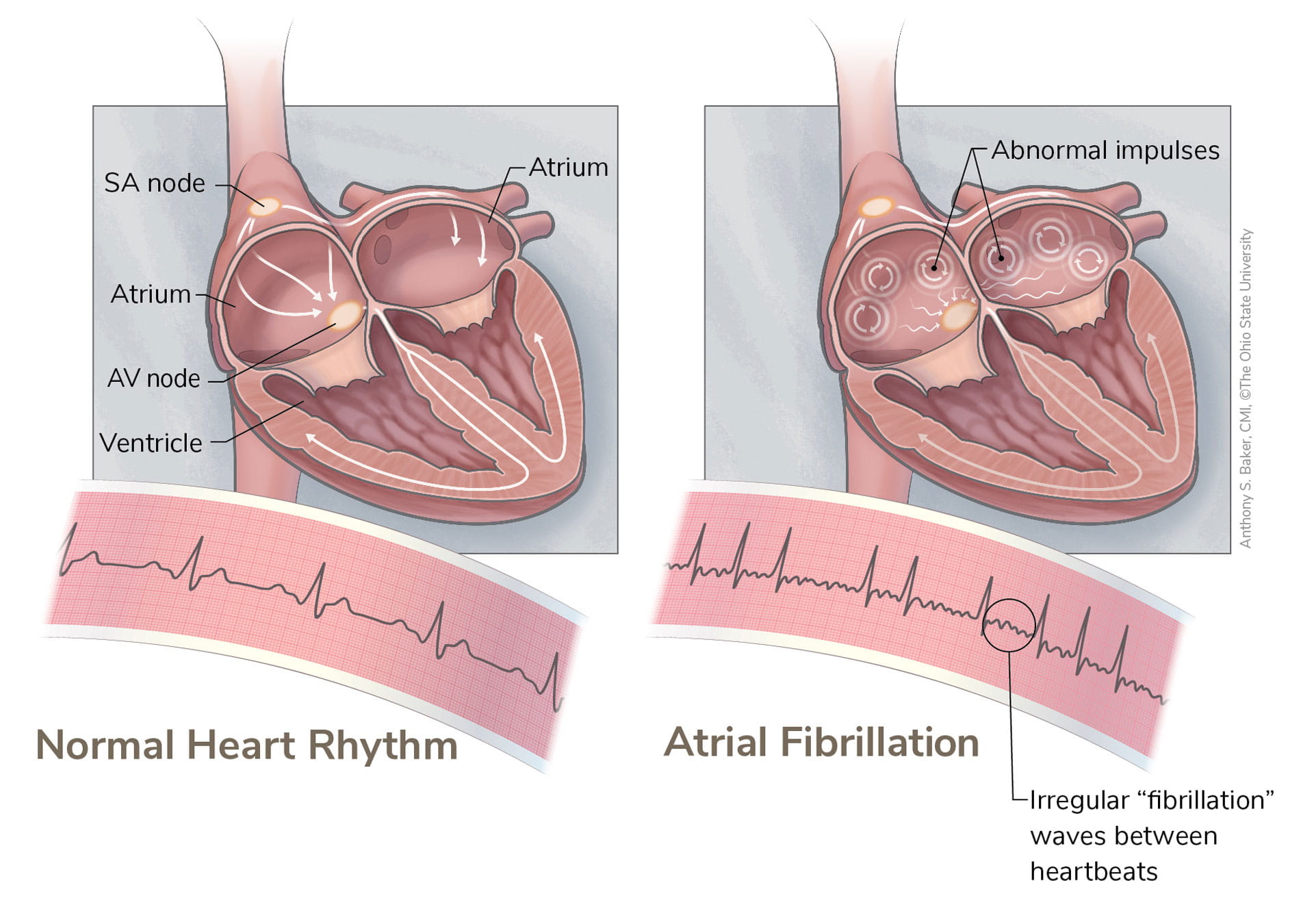
Ablation uses heat to interrupt the abnormal electrical activity that sustains atrial fibrillation. It’s a common procedure considered the standard of care in the treatment of AFib. Dr. Hummel and his colleagues observed that while they could successfully treat some patients by using the well-defined technique of eliminating triggers from the pulmonary veins, this approach was inadequate for others.
“For more than a decade, there was substantial controversy over whether or not there were sites outside the pulmonary veins that sustained AFib,” Dr. Hummel says. “The topic was of little interest to the world at large, but it was a burning issue within my field. Based upon existing studies in animals by other investigators, we suspected that if these sites did exist, we could target them and make them electrically silent, effectively eliminating AFib in many patients.”
The debate over whether or not these sites exist in humans ended in a laboratory on Ohio State’s campus, where Fedorov and his team developed a novel human heart model that provides a rare glimpse into the inner workings of the heart.

Visitors who don’t know that the heart can beat outside of the human body are in for a shock when they visit the Fedorov Lab. On any given day, they may find a glass chamber containing a recently donated human heart perfused with (bathed in) a warm oxygenated solution.
This solution stimulates blood flow, allowing the heart to beat for at least 12 hours with the same rhythm as when it was inside the donor’s body.
Many of these hearts come to the Fedorov Lab from patients undergoing a heart transplant at the Richard M. Ross Heart Hospital at The Ohio State University Wexner Medical Center who’ve elected to donate their diseased heart to science, while some hearts are donated through Lifeline of Ohio.

Once the donated heart is resuscitated, Fedorov and his team place atrial tissue in a perfusion bath surrounded by four ultra-sensitive infrared cameras. They then inject fluorescent dye that can sense electrical activity straight through the atrial wall.
The combination of the dye and the infrared light reveals an unprecedented level of information about the structure and electrical function of the heart. While current clinical imaging displays 200 recordings across the heart, Fedorov’s imaging system displays 40,000.
“Essentially, this imaging allows us to see how electricity spreads across the structure of the human heart in 3D,” Fedorov says.
“It confirmed the presence of something we were already seeing in animal models: re-entrant drivers in the atria. These are small sites outside the pulmonary veins where we can observe the abnormally fast electrical activity that sustains AFib.”
Published in European Heart Journal in 2015, Fedorov’s findings definitively established the existence of re-entrant drivers and ushered in a new era for translational patient care.
Translating research into results
Dr. Hummel remembers meeting Fedorov in 2013 at a talk Fedorov delivered on disease of the sinus node, the natural pacemaker of the heart. Hummel immediately recognized the translational potential of the imaging technology Fedorov was describing, calling it a “feather in the cap of Dr. Fedorov and Ohio State.” From that day on, a fruitful collaboration emerged.
With the exception of one overseas medical facility, Ohio State is the only health center in the world employing this technology in human hearts. By inducing AFib in a donated heart, Fedorov can gather information that illuminates a better treatment strategy for patients.
One of these patients, Richard “Steve” Hines, ended up in the ICU after a routine physical revealed he was experiencing atrial fibrillation. “There would be two or three regular beats then it would be a real low one or a real quick one, just all over the charts,” remembers Hines. According to Dr. Hummel, Hines was a “poster child” for the type of recurrent, 24/7 atrial fibrillation that many of his patients experienced.

“By giving us greater clarity into where the drivers of AFib might be, Dr. Fedorov is helping us figure out how to deliver better therapy to the types of patients we treat every day,” Dr. Hummel says.
Two weeks after Hines’ first ablation, he learned that the procedure failed to restore his heart to a normal rhythm. His second ablation also failed to correct the problem. For his third ablation, Dr. Hummel used the 3D imaging technology developed by Fedorov to guide him.
“He explained to me that with their new procedure, they could pinpoint the problem areas and the chances of getting it was a lot better,” Hines says. “It was pretty exciting because you know that whether it does or doesn’t work, they’re going to learn something from it. I’m fortunate to be with people who think outside of the box.”
The third time was the charm for Hines.
“If you look at our data on the patients we’re treating using the information gleaned from this new technology, the success rates at a year and a half post-ablation run around 75-78%, which is significantly better than the national average of 40-60%,” Dr. Hummel says. “Our data is still preliminary, but it’s showing signs of great promise.”
To date, Fedorov and Dr. Hummel have co-authored close to 30 research articles, several of which are focused on what re-entrant driver sites look like anatomically with MRI imaging. In one publication, they reported finding that re-entrant drivers aren’t always a closed loop, as previously understood, but are instead more like hubs of electrical activity that resemble tornados.
In another joint study, they established that the right atrium is three times more sensitive than the left to adenosine, a biochemical produced during times of metabolic stress – like after a heart attack or bypass surgery. During clinical tests, the research team found that injecting adenosine affects cardiac electrical activity, making the tornados that signal atrial fibrillation even more apparent. By injecting adenosine intravenously before ablation, they were able to improve the identification of ablation targets by 80% for patients enrolled in a clinical trial.
“Everyone likes to talk about translational research, but it’s rare to find such a straight line of connection between two labs,” Dr. Hummel says. “It’s been an exciting partnership that relies on the strength of a much larger team than the two of us.”
New frontiers in AFib
Together with the colleagues in their respective labs and collaboration with the Bob and Corrine Frick Center for Heart Failure and Arrythmia, Fedorov and Dr. Hummel are writing a new chapter in the study of atrial fibrillation. Despite their accomplishments, they’re quick to point out that they have just as many questions as answers.
“We’re still in the process of learning what makes some regions of the heart more susceptible to becoming a hub for AFib,” Dr. Hummel says.
“We need to find out what makes some driver sites more and less important, not just electrically but anatomically. If two patients have a different number and location of driver sites, can we determine that further ablation is wasted effort in one case but not the other?”
Fedorov and Dr. Hummel are also keen to link specific patient characteristics to AFib. In the near future, they’ll release the results of a study that demonstrates key differences in treatment response by biological sex. More narrowly, they’re working to identify patterns of AFib in patients with specific diseases. “To provide an example, we already know that sleep apnea, a risk factor for AFib, leads to AFib in the right atrium more frequently than the left, but the opposite is true with hypertension,” Fedorov says.
On the other hand, Dr. Hummel often sees entire families who suffer from AFib despite a healthy lifestyle and the absence of related diseases. “There is still so much we don’t know about the mechanisms that drive this condition,” he says.
Ultimately, they aim to develop therapeutic drugs, ablative therapies and prevention strategies to target AFib in individual patients. “The more we can understand AFib in the context of specific health conditions, the more we can tailor our treatments to individual patients,” Fedorov says. “This is the next frontier in patient care.”
Currently, he and Hummel are focused on identifying molecular targets for drug therapy. “It may be that different cells have features that make them uniquely targetable, but we’re not quite there yet,” Hummel says.

Ingredients for success
It’s not uncommon for Fedorov to spend the night in his lab. When a donated heart arrives, he and his team frequently work 24-hour shifts to gather and analyze data.
Their work is relentless and rewarding in equal parts. “What we do requires sacrifice, but it’s worth doing to benefit cardiac patients,” Fedorov says. “Dozens of people are involved in every research project we undertake, and their hard work has gained international recognition.”
Like Fedorov, Dr. Hummel draws strength from the mission of providing relief to as many patients with AFib as possible.
“I don’t want to create false hope,” he says. “While a good 55% of patients will be completely healed from a quick, routine procedure, we have to acknowledge that 10% aren’t treatable at all, even with our best efforts. The remaining 35% are somewhere in the middle. If we can continue to improve the way we map and target re-entry sites, we can help this group.”
The Ohio State Wexner Medical Center has all the right pieces for success in the battle against AFib: a large group of electrophysiologists, a world-class physiologist who specializes in cardiac arrhythmia, a one-of-a-kind heart model and a deep bench of experts in cardiac genetics and imaging.
“We’re well positioned to accelerate the process of translating basic science into clinical studies and outcomes,” Dr. Hummel says. “That’s what gets me out of bed in the morning. Our work may not put aFib in the rearview mirror just yet, but it will help a lot of people.”
With plenty of unanswered questions before him, Fedorov anticipates many more nights working in his office and lab.

“When I think about my grandfather in that hospital, or when I think about the loved ones so many families like mine have lost, that keeps me going through the long days and nights. We have to press on.”

Take charge of your heart health
Learn more about the causes of atrial fibrillation (AFib) and treatment options available at Ohio State.
Take charge today